The Molecular Magnetism Group in Manchester involves Professors David Collison, Eric McInnes and Richard Winpenny, along with Doctors Nicholas Chilton, Grigore Timco and Floriana Tuna. The group has great synthetic expertise, developing new methods for making molecular nanomagnets by studying new ligands, new reaction conditions and new combinations of metal centres. This is combined with a strong effort in physical studies. In Manchester these concentrate on magnetic measurements and EPR spectroscopy, where Collison and McInnes are the UK leaders in EPR of transition metal compounds in general. The group also has a strong computational and theoretical aspect, involving simulations of magnetic and spectroscopic data along with multi-configurational ab initio calculations.
With collaborators throughout the world we look at further techniques including inelastic neutron scattering, very high frequency and field EPR spectroscopy, very low temperature magnetic measurements, and transmission diode oscillators. We work closely with outstanding theoretical physicists using their expertise to interpret our data and our expertise to reproduce in molecules what they've described in Hamiltonians. We are beginning work with groups elsewhere in Manchester, looking at creating hybrid devices involving molecular nanomagnets with low-dimensional carbon.
We have also collaborated with synthetic chemists who are expert in other areas, e.g. Prof David Leigh FRS for supramolecular chemistry and Prof Stephen Liddle and Dr David Mills for very air-sensitive compounds, and Professors Collison and McInnes also run the EPSRC-funded National EPR Facility in Manchester.
Our group investigates the properties of chromium based heterometallic rings for a range of applications, although primarily their magnetic properties. Adjacent metals in the eight membered ring interact antiferromagnetically, such that {Cr7Ni} (Cr(III), d3, S = 3/2; Ni(II), d8, S = 1) results in a net spin of S = 1/2. In an applied magnetic field, the mS = ±1/2 state splits according to the Zeeman interaction, and it has been proposed that the resultant states could represent the required two-level system of a quantum binary digit (qubit). Work within our group aims to construct such a qubit through the physical coupling of two rings.
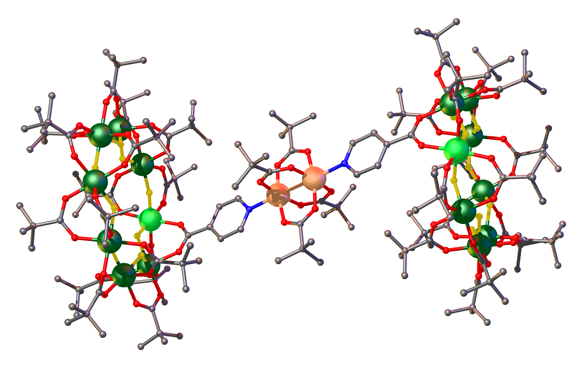
Figure: Crystal structure of two isonicotinic acid functionalised {Cr7Ni} rings linked along the axis of a copper pivalate dimer.
Finite chromium chains, commonly referred to as chromium horseshoes, can be considered as derivatives of both homo- and heterometallic chromium rings; removal of chromium/divalent metal sites and incorporation of an appropriate cation (e.g. ammonium) results in the formation of horseshoe supramolecular assemblies. The chains present novel and interesting chemistry due to the fact that the horseshoe cages can be considered as stable polyfluoride donor ligands; thus providing a pathway—which has been utilised with great success—to reactions involving the 4f (lanthanide) metals, and therefore extending the chemistry beyond the d- and p-blocks.
Magnetic properties derive from the both the spin and orbital angular momenta of electrons. The 4f elements (lanthanides) can possess ample amounts of both simultaneously, which can give rise to a high degree of magnetic anisotropy (good for SMM behaviour). In other cases, such as in Gd(III), we can have a large spin with minimal orbital angular momentum (good for MCE behaviour).
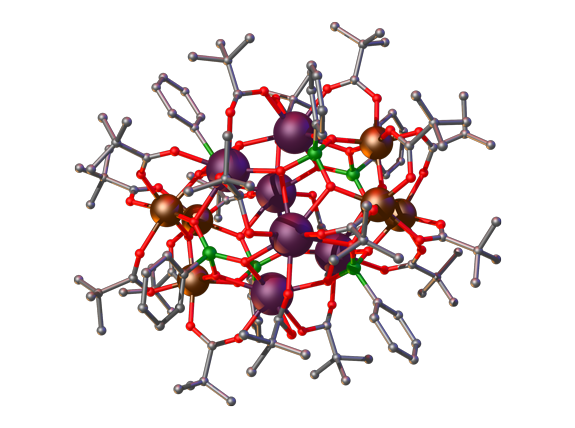
Figure: An {Fe6Gd6} Wells-Dawson cage formed by the bridging action of pivalates and phosphonates.
We are investigating both pure 4f cluster compounds and clusters containing a mixture of 4f and 3d metals (the 3d metals are either paramagnetic or diamagnetic and may be redox active), in order to find novel structures exhibiting single-molecule magnet and magnetocaloric effects.
Modelling the magnetic behaviour of systems containing ions with a large magnetic anisotropy induced by spin-orbit coupling remains a significant challenge. Work within the group aims to help further our understanding of these systems, both through advanced theoretical calculations (particularly on lanthanide compounds), and through practical spectroscopic investigations of low-nuclearity complexes, focusing particularly on multi-frequency electron paramagnetic resonance (EPR), inelastic neutron scattering (INS), and magnetic studies.
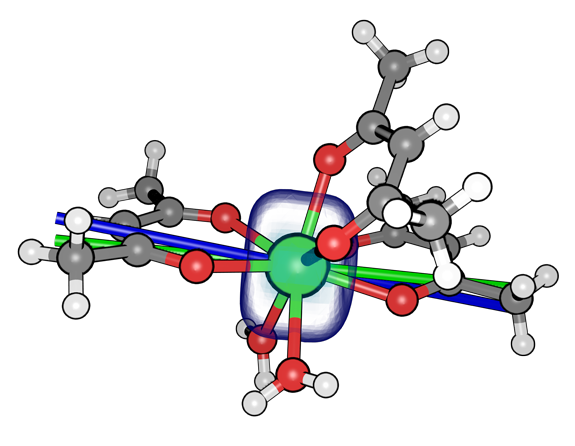
Figure: Modelling the anisotropy of a dysprosium complex using an electrostatic approach.
Current work aims to establish the relationship between the electronic properties and geometric structures of such highly anisotropic complexes in the hope of determining guidelines for the rational design of target materials.